Hydrogel Stiffness
Stiffness and Tissue Function
The mechanical properties of the extracellular matrix are important for the regulation of cell behavior (1) and other tissue functions. Therefore, matrix stiffness is an important experimental parameter for in vitro 3D cell cultures.
3-D Life Hydrogels can be formed with stiffnesses of up to a 10 kPa dynamic shear modulus. Therefore, the stiffness of a broad range of mammalian tissues (2) can be modeled.
Both, the thio-reactive polymers and the thiol-containing crosslinkers of the 3-D Life System, which are the basic reagents for forming hydrogels, are supplied as highly concentrated stock solutions. The stiffness of the hydrogels can easily be varied by adjusting the final concentration of these two reagents. Assistance for setting up pipetting protocols for hydrogels with varying stiffness can be obtained by utilizing a Hydrogel Calculation Tool.
Stiffness of ToLuminate Photogels
ToLuminate Photogels (PG series) are hydrogels crosslinked upon illumination with light at 360-405 nm, utilizing the cell-compatible thiol-ene chemistry (3).
It works by combining thiol-modified crosslinkers with norbornene-modified dextran (N-Dextran) and the photoinitiator LAP (Lithium phenyl-2,4,6-trimethylbenzoylphosphinate). Light activates LAP, which in turn activates the thiol groups of the crosslinker. The activated thiol groups will subsequently covalently bind to the norbornene groups of N-Dextran, forming a stable polymer network. The crosslinking reaction can be done in the presence of thiol-modified peptides, which simultaneously will also be attached to the polymer network utilizing the same chemical reaction.
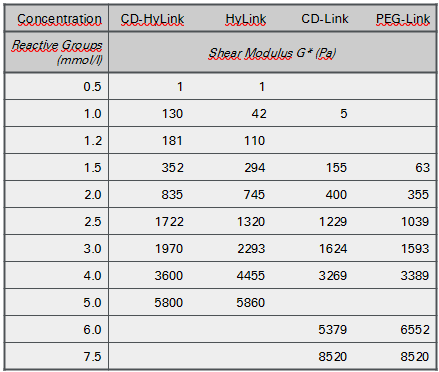
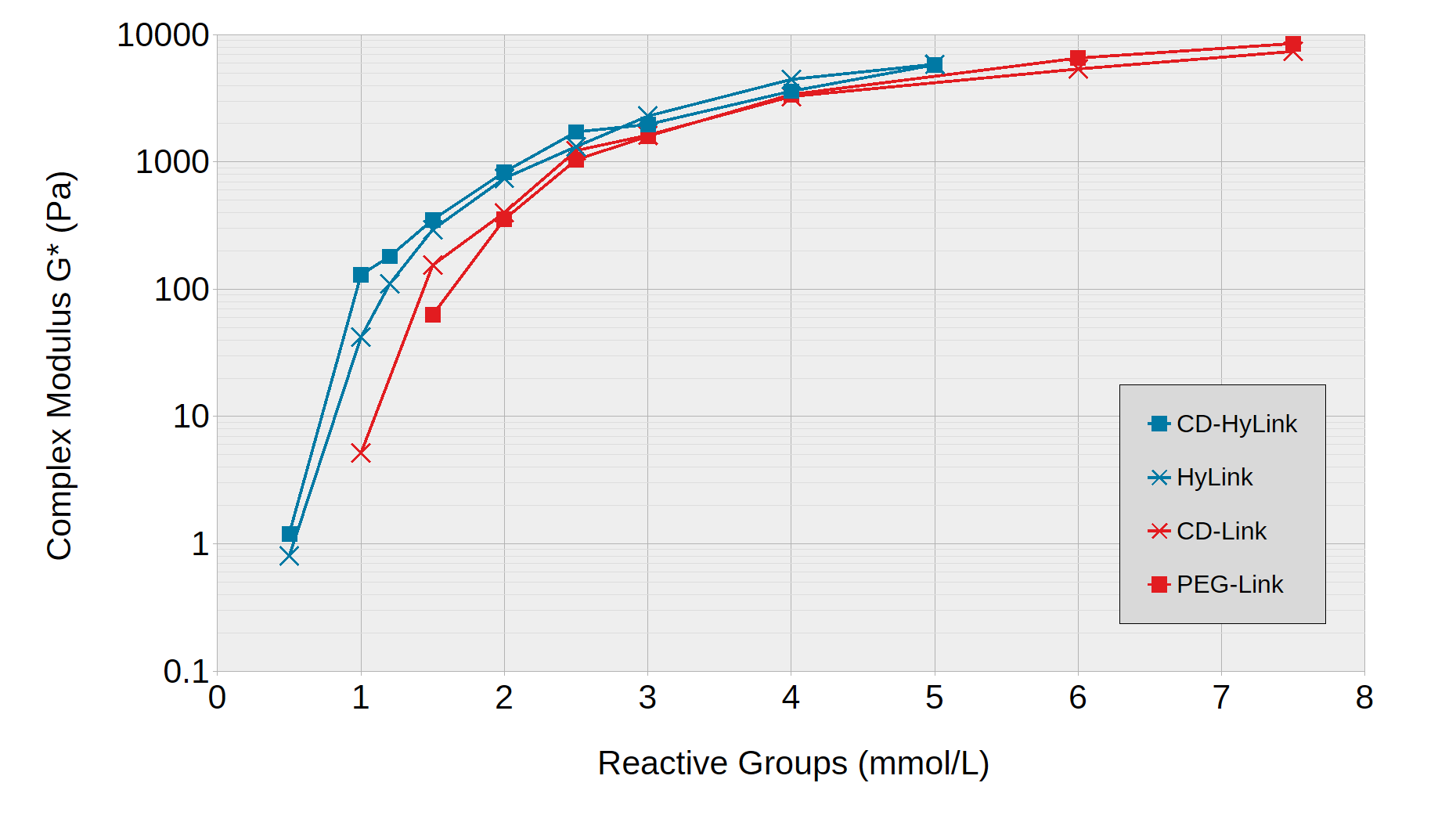
ToLuminate Photogels can be formed at varying stiffnesses by adjusting the concentrations of crosslinkers and of N-Dextran. Figure 1 shows the stiffnesses obtained when N-Dextran was crosslinked with the hyaluronic acid (HA)-based crosslinkers HyLink and CD-HyLink and with PEG-based crosslinkers PEG-Link and CD-Link.
The HA-based crosslinkers were more effective as crosslinkers, forming slightly stiffer gels at the same concentration, compared to PEG-based crosslinkers. Gel formation started with very soft hydrogels at reactive group concentrations around 1 mmol/L. Stiffnesses with a Complex Modulus of more than 5000 Pa were obtained at reactive group concentrations of 5 mmol/L for the HA-based hydrogels and of over 8000 Pa at 7.5 mmol/L for the PEG-based hydrogels.
Stiffness of 3-D Life Hyaluronic Acid-based Hydrogels
3-D Life Hyaluronic Acid-based Hydrogels (HA series) provide a natural extracellular matrix component, Hyaluronan, as a major component of 3-D Life Hydrogels.
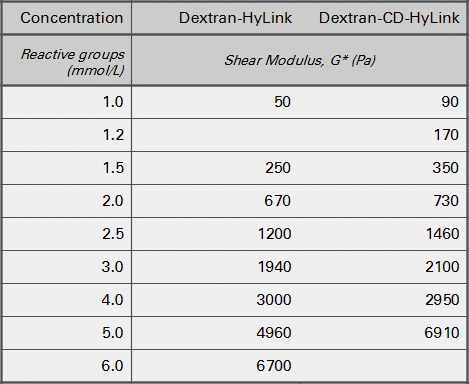
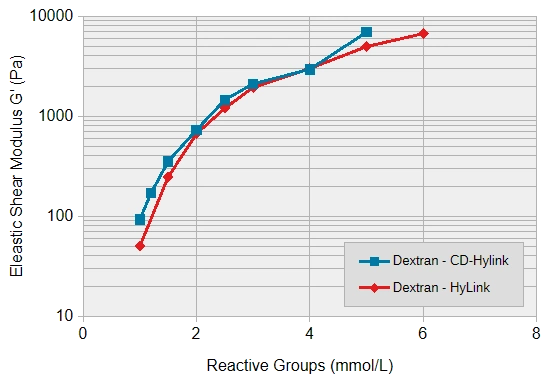
HA hydrogels can be used in a broad stiffness range from soft to medium stiff and the rate of gel formation is slighly faster than the rate of gel formation of slow-gelling hydrogels (SG series). Figure 2 shows the stiffnesses obtained when SG-Dextran was crosslinked with the HA-based crosslinkers HyLink and CD-HyLink and when SG-PVA was crosslinked with HyLink.
Gel formation was detected at crosslinking concentrations of 1 mmol/L and higher. At a concentration of 1 mmol/L of each reactive group, both types of crosslinkers develop a very low hydrogel stiffness (a shear modulus (G*) below 100 Pa). At higher crosslinking concentrations the stiffness increases roughly in a similar pattern by both crosslinkers up to several thousand Pa at 5-6 mmol/L.
Stiffness of 3-D Life Slow-Gelling Hydrogels
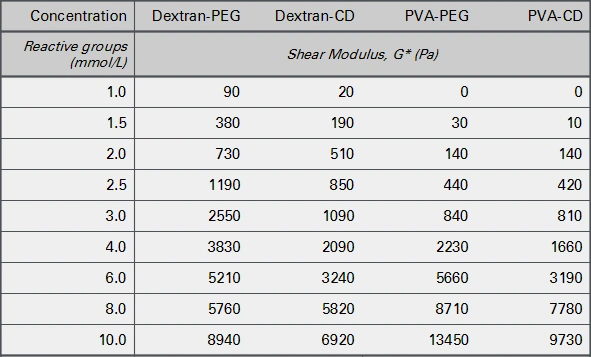
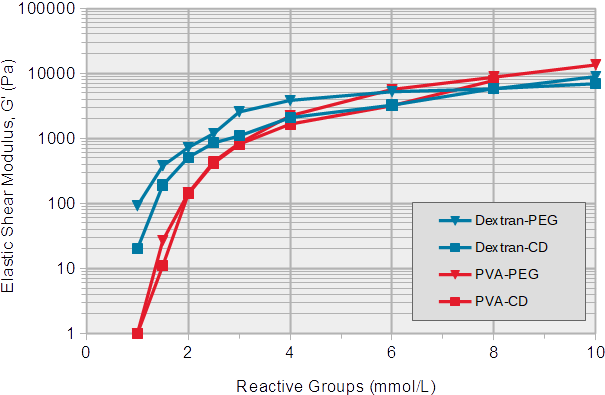
Slow gelling hydrogels (SG series) are used for a broad range of applications, with a soft to medium high stiffness, because the gelation time is long enough to easily handle the solutions. Figure 3 shows gel stiffnesses and corresponding concentrations of reactive groups of the polymers.
Gel formation starts at a concentration of 1 mmol/L reactive groups with a very low stiffness of 20-100 Pascal (Pa). With increasing concentrations of reactive groups, the stiffness of the hydrogels increases up to 10 kPa at a concentration of 10 mmol/L reactive groups. Below 4 mmol/L, the PVA-based hydrogels were softer than the dextran-based gels. The shear loss modulus (G''), a measure for the viscosity of the hydrogel, remained very low (below 2% of the G') in all combinations (data not shown).
Stiffness of 3-D Life Fast-Gelling Hydrogels
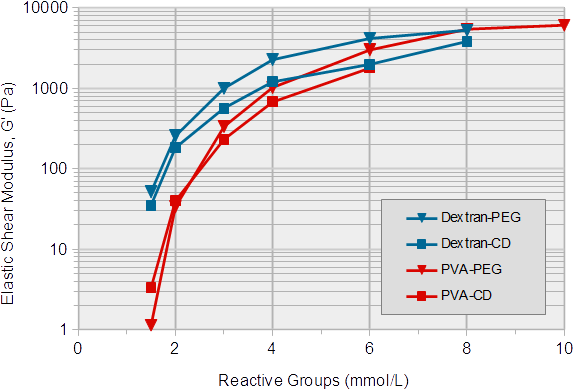
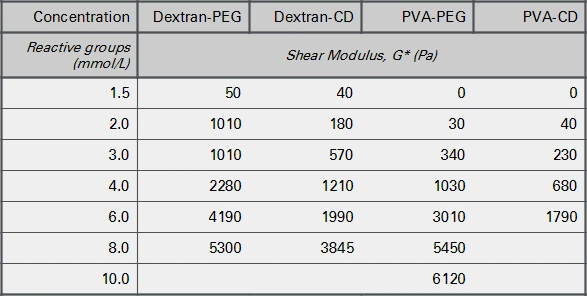
3-D Life Fast Gelling Hydrogels (FG series) are mainly used for the generation of soft hydrogels, since at higher stiffnesses, the gelation time becomes too short for a practical handling. Exceptions are automated pipetting and bioprinting applications. Figure 4 shows the stiffnesses of hydrogels formed at increasing concentrations of fast reacting polymers (FG series) and crosslinkers.
At a concentration of 1.5 mmol/L of each reactive group, all polymer combinations develop a very low hydrogel stiffness (an elastic shear modulus (G') below 100 Pa). This is markedly softer than the stiffnesses obtained from slow gelling gels at the same concentrations (compare Fig. 1). Below 6 mmol/L, the fast-gelling PVA-based hydrogels were softer than the fast-gelling dextran-based gels. At higher concentrations the stiffness increases up to 4-5 kPa. The shear loss modulus (G''), a measure for the viscosity of the hydrogel, remained very low (below 2% of the G') in all combinations (data not shown).
Literature
- Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008 Apr; 47(4):1394-1400.Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008 Apr; 47(4):1394-1400.
- Chen WLK, Simmons CA. Lessons from (patho)physiological tissue stiffness and their implications for drug screening, drug delivery and regenerative medicine. Adv Drug Deliv Rev. 2011 Apr 30; 63(4-5):269-276.
- Hoyle CE, Bowman CN. Thiol–Ene Click Chemistry. Angew. Chemie. 2010:49 1540-1573.